Stemson Therapeutics Uses Induced Pluripotent Stem Cells to Regrow Hair Follicles
I wanted to talk about a start-up biotech company based on California called Stemson Therapeutics, founded by Dr. Terskikh and Dr. Hamilton in 2018. Stemson Therapeutics, amongst many companies concerning stem cell replication for hair follicles, is in the works for finding a solution to create unlimited supply of donor hair by creating hair-inducing derma-papilla cells using induced pluripotent stem cells (iPSC). Our best bet right now is through stem cell multiplication and having the ability to have an infinite supply of hair follicles, which would cure baldness.

In a recent presentation at the annual meeting of the International Society for Stem Cell Research (ISSCR), Stemson Therapeutics revealed their study which involved the human stem cells being combined with mice epithelial cells and attached to a 3-D biodegradable scaffold, made from similar materials to dissolvable stitches. The scaffold acts as the growing environment for the derma- papilla cells as well as controlling the direction of hair growth and enables the stem cells to integrate into the skin. Now, they still have to overcome the task of using only human cells, which is currently being researched.
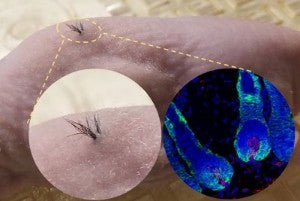
Below is an image of hair growth in nude mice transplanted with human iPSC-derived derma-papilla cells that were combined with mouse epithelial cells inside a biodegradable scaffold. The left image shows hair follicle growth on the mouse and the right is a fluorescent microscopy image of hair follicles under the skin; the blue is the cell nuclei, the green is the epithelial cells and the red is human derma-papilla cells.
The interesting part is that if Stemson Therapeutics is able to use human epithelial stem cells with the derma papilla cells to create hair follicles instead of with mice, they would be capable of allogenic transplantation in humans. I covered this similar topic last time regarding Seoul National University Hospital and their research on dendritic cells and ultraviolet B irradiation. But if Stemson Therapeutics is able to overcome this, hair follicles from a different donor or source would be possible to transplant without having to harvest the patient’s own cells.
The other interesting aspect is that in past research and studies, new hair growth was underneath the skin which this new concept produced hair follicles above and through the skin barrier.
The process, at least on paper, seems to be fairly straight forward. According to Stemson Therapeutics, they would simply just need to draw blood to receive the cells which would then be converted into induced pluripotent stem cells, which then would convert into human derma-papilla cells and epithelial cells.
Obviously, we still have a long way to go, and until one of these companies actually can show a bald man with a full head of hair, it’s all talk for now. It’ll be years before anything commercializes as it still has to go through the rigorous human clinical trials if everything else succeeds.
However, another interesting thing to add about this company is that they just secured a multi-million dollar investment from Allergan, which is a global pharmaceutical company, so that they can help advance Stemson Therapeutics’s final product development in preparation for human clinical trials. This is big news and Allergan sees the strong potential in iPSC’s not just for hair follicle replication but in regenerative medicine therapies in the future.

Hair may show up slight, yet you likely won’t go uncovered. The condition is entirely reversible. When the activating occasion is dealt with, your hair may begin developing back following a half year.Visit buy medication online for more information.
Thank you for providing such valuable information. all have had so much learning from this post.